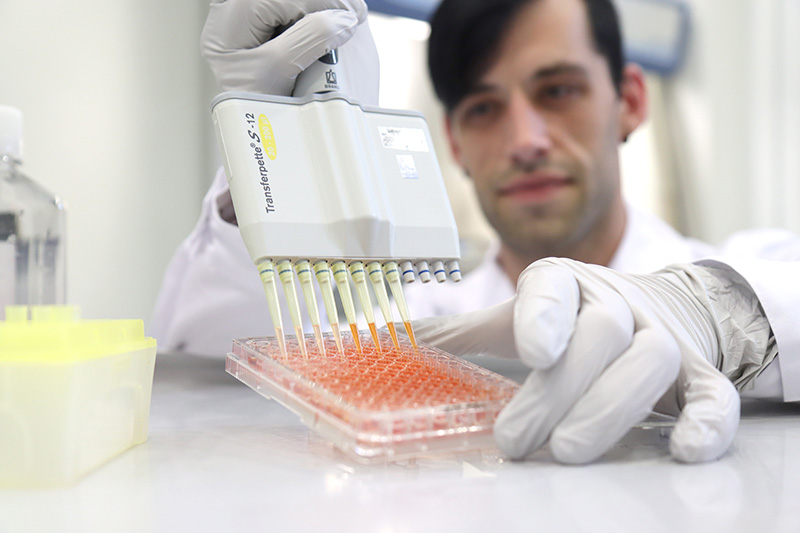
Dr. Thomas Dauben, head of the OMPG biology laboratory in Rudolstadt, during a cytotoxicity test. The different coloration on the microtiter plate indicates whether the cells are doing well. If the color is rather pale, their vitality is restricted by harmful substances. (Image rights: OMPG / Steffen Beikirch)
At OMPG in Rudolstadt, you can also feel that a lot is happening in this area. The test laboratory offers biological testing of medical devices and is accredited for this by the German Accreditation Body (DAkkS) and the Central Authority of the Federal States for Health Protection with regard to Medicinal Products and Medical Devices (ZLG). “These tests are used to assess the biocompatibility of medical devices and are a prerequisite for their approval,” says Laboratory Manager Dr. Thomas Dauben. The MDR regulation requires this proof for all materials with which patients, medical staff or other users may come into contact.
Three test methods are used to assess biocompatibility: testing for cytotoxicity, skin irritation and sensitization. “Manufacturers usually request all three tests from us as a package,” reports Dauben. However, OMPG customers most frequently order the cytotoxicity test in accordance with DIN EN ISO 10993-5. “This is the first and most important test in the biocompatibility assessment of medical devices. Only if there are signs of problems here is it worth going into more depth with the other two significantly more complex tests,” says the laboratory manager.
When testing for cytotoxicity, an extraction medium is added to the medical device to dissolve out potentially harmful substances. The extract is applied to living cells in various dilutions. After a certain incubation period under detailed conditions, the cells are then examined by means of a biochemical reaction to determine whether their growth or vitality has been restricted by harmful substances.
While this test has always been cell-based, the two further tests are usually carried out in conjunction with animal experiments. Not so at the OMPG: the Rudolstadt laboratory uses a method with three-dimensional human skin models to test for skin irritation. The OMPG is also currently establishing animal-free methods for testing for sensitization through allergenic potential, which should be available soon.
Meanwhile, an external round robin test has now been offered for the first time for the cytotoxicity test, in which the OMPG participated. “In the past, laboratories had to make their own efforts to carry out comparative tests with other service providers,” says Dauben. This is why the round robin test organized for the first time by the Johner Institute (Constance) with 18 laboratories, an external task and an independent external assessment is to be rated significantly higher. “The OMPG successfully passed the round robin test. This result is very important for our quality assurance and confirms that our processes are stable and guarantee reliable, reproducible results,” says Dauben.
Bei der Prüfung auf Zytotoxizität wird das Medizinprodukt mit einem Extraktionsmedium versetzt, um potenziell schädliche Stoffe herauszulösen. Der Extrakt wird in verschiedenen Verdünnungen auf lebende Zellen aufgebracht. Nach einer bestimmten Inkubationszeit unter detailliert vorgegebenen Bedingungen werden die Zellen dann mittels einer biochemischen Reaktion darauf untersucht, ob sie durch schädliche Substanzen in ihrem Wachstum oder in ihrer Vitalität eingeschränkt worden sind.
Während diese Prüfung schon immer zellbasiert durchgeführt wurde, finden die beiden weiterführenden Tests in der Regel in Verbindung mit Tierversuchen statt. Nicht so bei der OMPG: Das Rudolstädter Labor verwendet für die Prüfung auf Hautirritation eine Methode mit dreidimensionalen humanen Hautmodellen. Für die Untersuchung auf Sensibilisierung durch allergenes Potenzial etabliert die OMPG derzeit ebenfalls tierversuchsfreie Methoden, die bald zur Verfügung stehen sollen.
Unterdessen wurde für den Test auf Zytotoxizität jetzt erstmals ein externer Ringversuch angeboten, an dem sich die OMPG beteiligte. „In der Vergangenheit mussten sich Labore hier selbst um Vergleichsprüfungen mit anderen Dienstleistern bemühen“, sagt Dauben. Daher sei der erstmals vom Johner Institut (Konstanz) veranstaltete Ringversuch mit 18 Laboren, einer externen Aufgabenstellung und einer unabhängigen externen Bewertung deutlich höher einzustufen. „Die OMPG hat den Ringversuch erfolgreich bestanden. Dieses Ergebnis ist sehr wichtig für unsere Qualitätssicherung und bestätigt, dass unsere Prozesse stabil laufen und dabei verlässliche, reproduzierbare Resultate garantieren“, so Dauben.



![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/b/b/csm_brandpruefungen-und-elektroanwendungen_7036bf8d6b.jpg)

![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/8/a/csm_biologische-pruefungen_b330c70d45.jpg)


