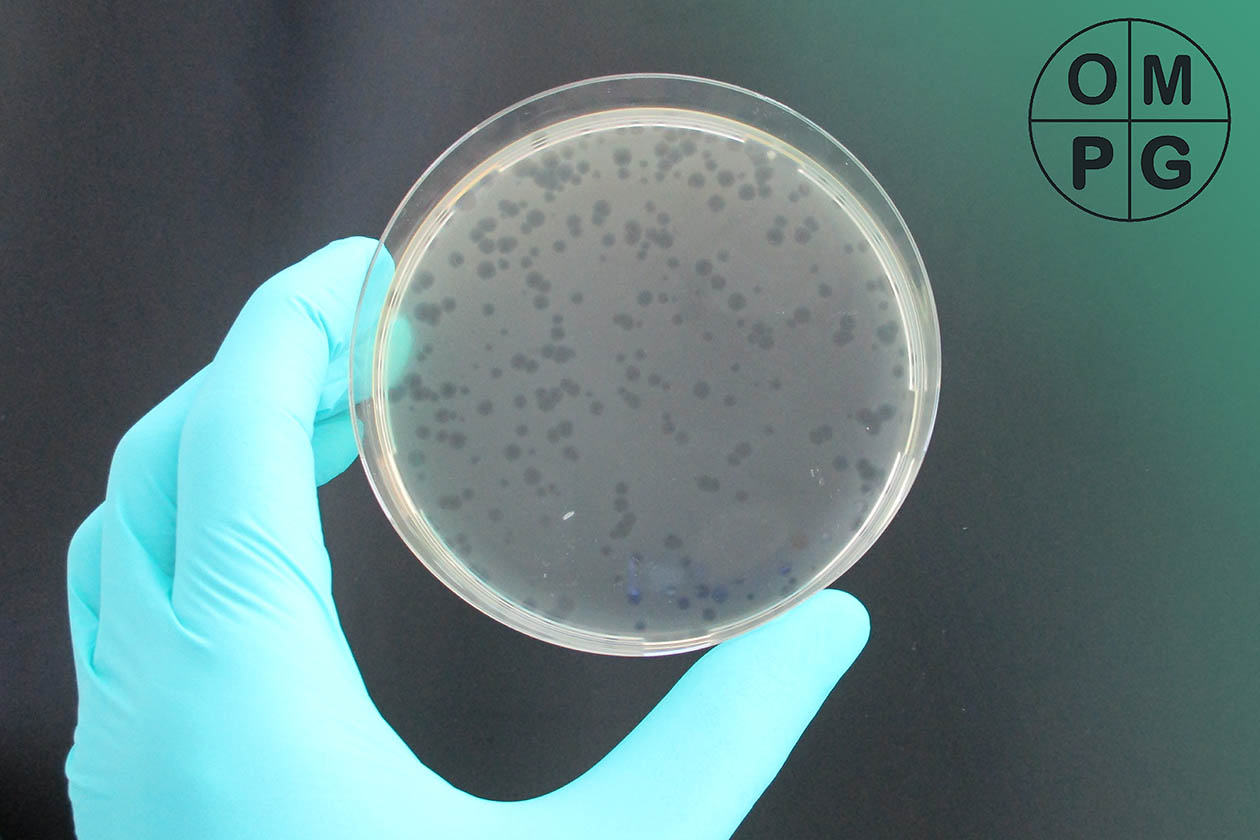
Plaque titer test of the bacteriophage phi6. (Image rights: OMPG)
International standards exist for testing antiviral materials, including ISO 18184 (Determination of antiviral activity of textile products) and ISO 21702 (Measurement of antiviral activity on plastics and other non-porous surfaces). Both standards are based on the influenza A virus (flu virus) and the feline calicivirus (FCV, cat flu) as test organisms.
“Since growing and cultivating viruses, especially human pathogenic viruses, in cell cultures is relatively time-consuming, the OMPG has significantly simplified antiviral efficacy testing by using bacteriophages,” says Dr. Thomas Dauben, Head of the Biology Laboratory. “Bacteriophages specifically infect bacteria and are therefore not only easier and quicker to cultivate than the standard viruses, but also pose no health risk to humans.”
The bacteriophage phi6 used by the OMPG belongs to the cystovirus genus and thus to the only bacteriophage family with a complete viral envelope. Due to this morphological characteristic, the bacteriophage also has a replication cycle that is comparable to that of other enveloped human pathogenic viruses, such as influenza A or SARS-CoV-2. “For this reason, it is ideally suited as a surrogate virus - i.e. a comparable alternative virus - for such human pathogenic viruses,” says Dr. Dauben. As a result, antiviral testing of textiles and plastics can now be carried out more easily and efficiently using the test method modified by the OMPG.
The further development of test methods by the OMPG also goes hand in hand with materials research at the Thuringian Institute for Textile and Plastics Research (TITK). The antimicrobial treatment of plastics has long been an important area of work there. Both specially developed antibacterial silver and zinc-based additives as well as antiviral coatings have already been investigated as part of research projects.
Background: Procedure for testing antibacterial and antiviral efficacy
The test methods are based on the respective antibacterial test standards for textiles (DIN EN ISO 20743) and plastics (ISO 22196): In each case, two types of bacteria, one gram-positive and one gram-negative bacterium, are used for testing. These are applied to the samples in a defined cell count and removed again after a contact time of 24 hours. The live cell count is then determined in comparison to a control material. The reduction in the bacterial count corresponds to the antibacterial efficacy. The OMPG is already accredited for these tests by the German Accreditation Body (DAkkS) and the Central Authority of the Federal States for Health Protection with regard to Medicinal Products and Medical Devices (ZLG).
Antiviral tests are carried out in the same way: A predetermined number of viruses are applied to the samples and removed after two hours (textiles) or 24 hours (plastics). The number of viruses is then determined using a plaque titer test by applying the viruses to a cell lawn in a decadal dilution series and counting the resulting cell-free zones, so-called “plaques”. From this, the virus titer on the samples can be calculated and the virus reduction can be determined in comparison to a non-antiviral control material. This reduction corresponds to the antiviral efficacy.



![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/b/b/csm_brandpruefungen-und-elektroanwendungen_7036bf8d6b.jpg)

![[Translate to English:] [Translate to English:]](/fileadmin/_processed_/8/a/csm_biologische-pruefungen_b330c70d45.jpg)


